
The Davis Lab enjoyed a holiday pot-luck style breakfast to commemorate a successful year of collaboration, research, and growth. We look forward to the new opportunities and continued developments in the new year!

The Davis Lab enjoyed a holiday pot-luck style breakfast to commemorate a successful year of collaboration, research, and growth. We look forward to the new opportunities and continued developments in the new year!


Nishant Sinha, PhD (Talk at SIG Neuroimaging Panel): Scalable quantitative methods to map epileptic networks and guide epilepsy surgery
Ryan Gallagher, MD student: Spatial Extent of Interictal Intracranial EEG Abnormalities Relates to the Focality of Epileptic Networks
Related paper: Quantifying interictal intracranial EEG to predict focal epilepsy.
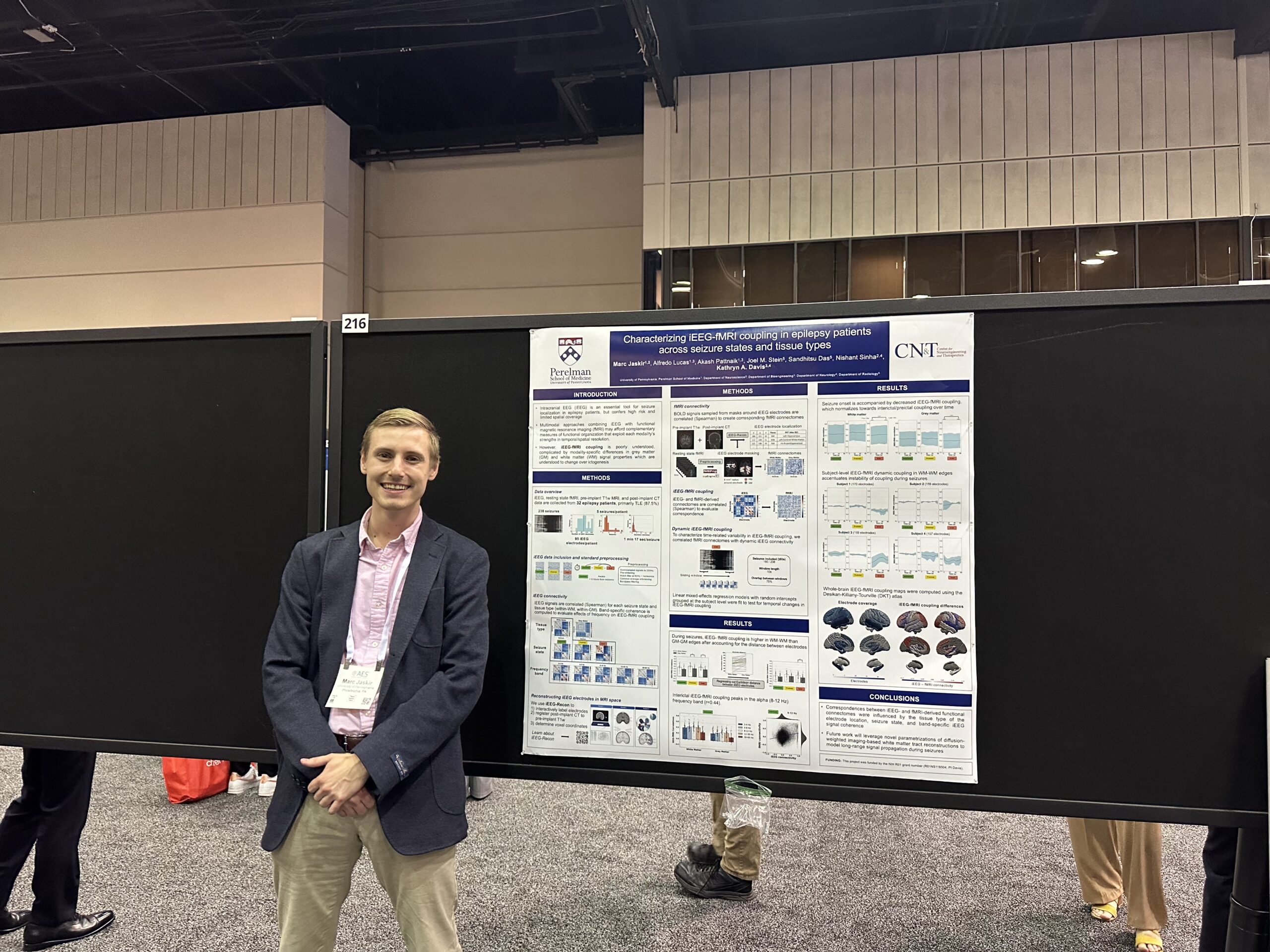 Marc Jaskir, PhD Student: Characterizing iEEG-fMRI Coupling in Epilepsy Patients Across Seizure States and Tissue Types
Marc Jaskir, PhD Student: Characterizing iEEG-fMRI Coupling in Epilepsy Patients Across Seizure States and Tissue Types
Rationale:
Intracranial EEG (iEEG) is an essential tool for seizure localization in epilepsy patients. However, iEEG is a highly invasive diagnostic procedure and its incomplete spatial coverage imposes limitations for whole-brain functional mapping approaches, which may reveal global network properties that are relevant to seizure onset and propagation. Multimodal approaches combining iEEG with functional magnetic resonance imaging (fMRI) may afford complementary measures of functional organization that exploit each modality’s strengths in temporal resolution and spatial coverage, respectively. However, iEEG-fMRI coupling is poorly understood1 and their relationship is further complicated by modality-specific differences between grey and white matter signal properties2,3 which are understood to change over ictogenesis. Therefore, we thoroughly characterized iEEG-fMRI coupling in epilepsy patients during different stages of ictogenesis and across different tissue types.
Methods:
Using resting state fMRI and interictal, preictal, and ictal iEEG data from 32 epilepsy patients, we evaluated differences in average iEEG-fMRI correlations and the associations between their graph theoretic properties (global efficiency) across different tissue types, seizure states, and frequency bands. We also used dynamic functional connectivity to elucidate seizure state-specific changes in iEEG-fMRI coupling over time.

Alfredo Lucas, MD/PhD Student:
Disparities and Algorithm Bias in Natural Language Processing of Epilepsy Outcomes
Exploring Cortico-cortical Evoked Potentials as a Marker of the Seizure Onset Zone
Guiding Intracranial EEG Implantation in Epilepsy Using Normative Brain Imaging
Improved Seizure Onset-Zone Lateralization in Temporal Lobe Epilepsy Using 7T Resting-State fMRI: A Direct Comparison with 3T
Multidien Cycles in Interictal Background Features of Long-Term EEG Recordings
Quantitative Intracranial EEG Abnormalities Across States of Consciousness in Drug-resistant Epilepsy
Role of Morphology in Interictal Spikes to Elucidate Seizure Generation
iEEG-recon: A Fast and Scalable Pipeline for Accurate Reconstruction of Intracranial Electrodes and Implantable Devices
Related papers:
Resting state functional connectivity demonstrates increased segregation in bilateral temporal lobe epilepsy
Dan Zhou, MD:
MEG Slow Wave Activity Associated with Brain Tumors in Patients with and Without Epilepsy
Rationale:
Epilepsy is prevalent in nearly half of patients with primary brain tumors. Recent evidence suggests that brain tumors can generate abnormal electrical activity in peritumoral tissue and affect local brain networks. Magnetoencephalography (MEG), a noninvasive imaging tool used for the functional mapping of eloquent cortex and localization of epileptogenic brain regions, has been used for cortical mapping prior to resective surgery. However, the evaluation of spontaneous interictal activity is often omitted. We aimed to characterize the interictal activity, including epileptiform discharges and slow activity, of patients who received presurgical MEG and compare the results between patients with and without brain tumor-related epilepsy (BTRE).
Methods:
We performed a retrospective analysis of the spontaneous interictal recordings in MEG during awake and sleep states in patients with primary gliomas who were recorded at the University of Nebraska Medical Center from July 2021 to March 2023. Focal slow wave activity (delta-theta band) was identified and processed for source localization using the equivalent current dipole method with Neuromag and Curry software. Dipoles were accepted when the goodness of fit was ≥80% and superimposed onto the individual patient’s brain MRI. A cluster was formed when ≥5 dipoles were localized closely together. Pre- and post-operative brain MRIs were used to determine tumor and resection sizes and locations, and the dipoles were then localized against the tumor resection sites.
 Nishant Sinha, PhD, a post-doctoral research fellow in the Center for Neuroengineering and Therapeutics, published a paper last month in Neurology that was featured as the cover article. The paper, titled “Intracranial EEG Structure-Function Coupling and Seizure Outcomes After Epilepsy Surgery”, examined the relationship between brain structure and function to determine the extent to which this relationship affects the success of the surgery in controlling seizures. This study showed that the strength of structure-function connectivity coupling may play a crucial role in determining the success of epilepsy surgery.
Nishant Sinha, PhD, a post-doctoral research fellow in the Center for Neuroengineering and Therapeutics, published a paper last month in Neurology that was featured as the cover article. The paper, titled “Intracranial EEG Structure-Function Coupling and Seizure Outcomes After Epilepsy Surgery”, examined the relationship between brain structure and function to determine the extent to which this relationship affects the success of the surgery in controlling seizures. This study showed that the strength of structure-function connectivity coupling may play a crucial role in determining the success of epilepsy surgery.
Click HERE to read the full article.
Read below to learn more about the projects that were presented at this year’s ICTALS conference by members of the Davis Lab!
Quantifying Seizure Spread Using Deep Learning Algorithms to Localize Seizure Onset
Andrew Y. Revell, Erin C. Conrad, Brittany Scheid, Brian Litt, Kathryn A. Davis
Intracranial EEG structure-function coupling predicts surgical outcomes in focal epilepsy
Nishant Sinha, John S. Duncan, Beate Diehl, Fahmida A. Chowdhury, Jane de Tisi, Anna Miserocchi, Andrew W. McEvoy, Kathryn A. Davis, Sjoerd B. Vos, Gavin P. Winston, Yujiang Wang, Peter N. Taylor
Sleep and Epilepsy
Erin C. Conrad, Andrew Y. Revell, James J. Gugger, Russell T. Shinohara, Brian Litt, Eric D. Marsh, Kathryn A. Davis
Epilepsy Surgery
John M. Bernabei, Nishant Sinha, T. Campbell Arnold, Erin Conrad, Ian Ong, Akash R. Pattnaik, Joel M. Stein, Russell T. Shinohara, Timothy H. Lucas, Dani S. Bassett, Kathryn A. Davis, Brian Litt
Read more about these projects HERE.
As a Research Assistant to Dr. Davis at Penn’s Center for Neuroengineering and Therapeutics, Hannah Gonzalez worked alongside Dr. Arnold to train a deep learning model to automate resection cavity segmentation on postoperative MRI of epilepsy patients to help physicians quantify removed brain structures.
The purpose of the project was to expand on a previous paper about Deep Learning-Based Automated Segmentation of Resection Cavities on Postsurgical Epilepsy MRI by adding a step in the data preprocessing and training of the model. After training 3 different models and running inference and the majority vote algorithm, it was determined that the Axial/Coronal model is the highest performer.
Future work in investigating the sagittal model to see how its performance can be improved.
Read more about this project HERE.
Penn students, many of whom joined the Davis Lab this summer, developed new tools and furthered our understanding of the relationship between neural structure and function in intractable epilepsy patients. Their research strives to improve presurgical localization of seizure onset, a result which could lead to a greater chance of seizure freedom for patients with epilepsy not fully managed by medications.
Penn Today highlighted this work in a new report, emphasizing the impressive contribution of the students to many projects in the Davis Lab. “They take an interdisciplinary approach, drawing from their expertise in imaging analysis, machine learning, network analysis, and signal analysis to solve a very relevant clinical problem,” Penn Today said of the students.
Read the full article by Brandon Baker in Penn Today HERE.
M.D., Ph.D. students John Bernabei and Andy Revell presented grand rounds for the Penn MSTP on Monday. Their lecture focused on the rapid emergence of AI in medicine and how tools currently being used by epilepsy researchers in the CNT and Davis Lab could one day change the fundamentals of clinical practice.
When applied effectively AI tools “can perform staggering computational tasks”, said Bernabei and Revell. In a clinical case study they presented, AI tools were used to sort through 5 million possible genetic variations in an infant presenting with seizures to identify the gene of interest– a time-sensitive feat which would have been impossible to perform manually. Cases such as this highlight an important role for these tools in medicine, they said.
However, Bernabei and Revell also noted that there are key limitations of AI. Machine learning algorithms, for instance, are largely a product of the datasets used to train them. The patterns in data AI is taught to recognize can sometimes be the algorithm’s own undoing. “When AI hears hoofbeats, it’s really only going to ever think of horses, and really never zebras. Humans are better at picking up zebras”, they explained.
Bernabei and Revell concluded that in a future almost certainly involving AI at the bedside, physicians should aim to integrate AI into their practice in a way that effectively leverages both computational tools and human medical expertise.
At this year’s annual meeting of the American Epilepsy Society, former CNT student and current Penn Epilepsy Fellow Erin Conrad, M.D., presented her soon-to-be published findings in a talk titled, “Does the Spatial Distribution or Frequency of IED Predict Seizure Onset Zone?”
The AES annual conference is the largest gathering on epilepsy in the world, and Conrad’s talk was part of an expert-led symposium on how interictal epileptiform discharges, or pathological patterns of brain activity between seizures, can be used to localize seizure onset.
Her findings have been recently accepted to Brain: A Journal of Neurology under the title, “The spatial topology of interictal spikes evolves over time and predicts the location of seizure onset”. To find out more about AES19 and Conrad’s talk, please visit the #AES19 website.
John Bernabei, current M.D., Ph.D. student, and Lohith Kini, M.D., Ph.D., were the primary co-authors on a new publication in Brain on Thursday. In their paper, the authors introduced and shared a set of network-based techniques aimed at predicting the most effective surgical resection zone in epilepsy patients.
Virtual resection, as the technique is called, has been shown to identify patients who are most likely to benefit from the removal of epileptic tissue. Within this patient group the authors hope their tools can specify the most important regions for removal, ensuring the highest probability for post-surgical seizure freedom.
Additionally, the authors show that in some cases tissues close to the seizure onset zone can work to dissipate, or “desynchronize” seizure activity from the start. Identifying these desynchronizing networks enables physicians to plan around them, avoiding their removal in a resection or ablation procedure.
Bernabei and Kini both contributed to this work as members of the CNT, a multidisciplinary environment that seeks to bridge gaps between fields in its research.
“We brought together senior faculty from Neurology, Neurosurgery, Radiology, and Bioengineering to guide a translational project using network neuroscience to guide patient care,” Bernabei says. “I’m fortunate to have mentors here who are dedicated to helping me achieve my goals as a physician-scientist in training.”
To read the full article in Brain click HERE.
Jennifer Stiso, a graduate student in the lab of CNT member Danielle Bassett, Ph.D., recently published findings in Cell Reports detailing how models of neural networks can be used to personalize brain stimulation therapies with the goal of improving memory. The modeling of neural networks using electrode data gathered during the treatment of epilepsy is an ongoing source of collaboration between members of the Bassett Lab and Davis Lab.
Penn Today highlighted this work on Thursday in an article explaining how brain stimulation therapies, such as those used in the treatment of neurological disorders like epilepsy, can be expanded upon to improve memory or restore motor function.
“This type of study is an important, early step towards developing a fast, generalizable model of an individual’s response to a specific stimulation therapy,” said Stiso.
Models of neural networks can give researchers unprecedented insight into neural function, and collaborations between the Bassett Lab and Davis Lab seek to use these models to develop novel therapies for a wide range of disorders.
Read the full Penn Today article HERE.