Penn students, many of whom joined the Davis Lab this summer, developed new tools and furthered our understanding of the relationship between neural structure and function in intractable epilepsy patients. Their research strives to improve presurgical localization of seizure onset, a result which could lead to a greater chance of seizure freedom for patients with epilepsy not fully managed by medications.
Penn Today highlighted this work in a new report, emphasizing the impressive contribution of the students to many projects in the Davis Lab. “They take an interdisciplinary approach, drawing from their expertise in imaging analysis, machine learning, network analysis, and signal analysis to solve a very relevant clinical problem,” Penn Today said of the students.
Read the full article by Brandon Baker in Penn Today HERE.







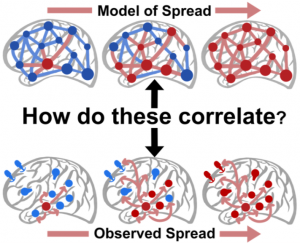
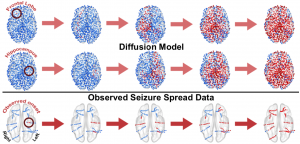

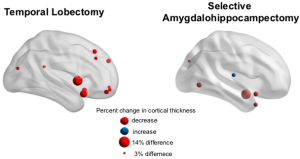

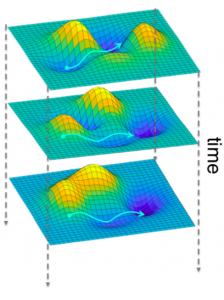


 The International Conference for Technology and Analysis of Seizures has invited Dr. Davis to speak at its September conference ICTALS 2019 at the University of Exeter. The theme of this year’s meeting is “The Epilepsy Journey: from first seizure to treatment and beyond”. A major focus of discussion will be how recent advances in our understanding of brain dynamics can make a difference for epilepsy patients at all stages of their journey. To find out more about the event, click
The International Conference for Technology and Analysis of Seizures has invited Dr. Davis to speak at its September conference ICTALS 2019 at the University of Exeter. The theme of this year’s meeting is “The Epilepsy Journey: from first seizure to treatment and beyond”. A major focus of discussion will be how recent advances in our understanding of brain dynamics can make a difference for epilepsy patients at all stages of their journey. To find out more about the event, click